![]()
On the Instability of Amalgams
- Increased mercury emission...
![]()
The symbiosis between the dental and industrial communities and their scientific journals
![]()
On the Instability of Amalgams...cont. -
Types of dental amalgams
Dental amalgams may be classified as copper-, conventional and non-gamma-2 amalgams. Non-gamma-2 amalgams are sometimes also called high copper amalgams (4).
Copper amalgams
Copper amalgams should not be mistaken for the high copper or non-gamma-2 amalgams described later.
The composition of copper amalgam is not regulated in any ISO standard. They consist of approx. 30% copper and 70% mercury. Sometimes small amounts of other metals such as cadmium, silver and zinc have been added - Neo-Silbrin 1,5% Cd, Cupromuc 0,6% Cd (3, 11, 12).
Copper amalgam is manufactured as small cylindrical pellets of metal. These are placed in an amalgam spoon and heated until small drops of liquid mercury are visible on the surface. This occurs at approximately 96 [ring]C (3), see Photo 1 and Photo 2.
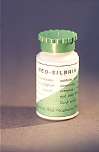

Photo 1 Photo 2 Click in the image to view full size! The mass is mechanically mixed in the spoon and after cooling somewhat it is placed in the cavity. Liquid mercury can be found in the filling 24h after preparation (3).
Copper amalgams are used primarily in the case of extensive caries attacks and cavities with poor retention in children but sometimes also in grownups. As early as 1890 Miller found copper amalgams to be cariostatic (12). Although statistics are lacking it is anticipated that copper amalgams have been used to a much lesser extent than conventional amalgams. They are regarded to be the most unstable of the three types corroding extensively. Sometimes this results in greenish teeth from copper-containing corrosion products (13). In animal experiments they were found to be 50 times as unstable as other types of amalgams (14).
Sauerwein proposes an alternative way of using copper amalgam (15). It is used as a "shell" in the cavity but the rest of the filling is made up by ordinary amalgam. The bad corrosion resistance, severe porosity and mechanical weakness can be limited in this way. The negative properties of copper amalgam are however such that the author recommends that it should no longer be used.
In a letter Prof. Trond Hegdahl, University of Bergen expressed his deep concern over the presence of cadmium in the copper amalgam Neo-Silbrin, proposing that this product should be withdrawn from the market (16). This letter was answered by NIOM, The Scandinavian Institute of Dental Materials, a body carrying out testing and certification of dental materials in Denmark, Finland, Iceland, Norway and Sweden. In this answer it is estimated that the intake of cadmium from this amalgam may be quite high and comparable to the intake via food. The intake of mercury can however be 315 times that from food in a worst case scenario, see Figure 1.
Despite the alarming facts about copper amalgam presented in this almost unknown answer by the head of NIOM at that time, Prof. Ivar A Mjør, no authorities in the other countries except Norway were informed (17). Not even the members of the NIOM board were informed, something that should take place according to NIOM.
Although the knowledge of the considerable instability of these amalgams with possible health effects in children, dental officials have withheld this information from the public debate.
Despite the fact it has been concluded that these amalgams are leaking mercury and sometimes cadmium to an extent where children can be subjected to toxic levels (17) they have been used in Sweden in the 1980's and in Norway in the mid 1990's (18).
Quite a number of adults in the Nordic countries have been exposed to copper amalgam as toddlers, children and teenagers.
Copper amalgam is still used in the former USSR, (19, 20). Two of the brands used in Sweden and Norway were manufactured in Germany. Chuev et al mentions that their copper amalgam TMAC-01 is analogue to a German copper amalgam manufactured by Becht (19). Strangely enough very little information on the use of copper amalgam in Germany has been found.
Skinner and Philips, the 1967 ed., mention 9 different brands of copper amalgam tested for composition (21). The investigation is provided by H.K. Worner and no further information on its publication is provided. An admixture of a conventional amalgam and a copper amalgam has been / is sold on the U.S. market (4, 22). This raises the question about the use of copper amalgam in the US.
Few researchers have done investigations regarding copper amalgam. Statistics on the use of these are hard to come by. Contacts with dental officials give the impression that these amalgams have not been used for a long time. After some investigation these statements have been proven wrong.
Conventional amalgams
Conventional amalgams have a long history and can be traced back to the fore-figure in dental science G.V. Black (23). In modern times the composition of the alloy-powder in amalgams was regulated in a standard from ISO (24). This standard is technically identical to the FDI World Dental Federation Specification No. 1. It is important to note that it is the composition of the alloy powder that is regulated - not the final filling. Stability criteria of the final product such as corrosion, emission of mercury vapor, etc. are not mentioned. The amount of mercury used to form the final amalgam mass is not regulated in this standard apart from demands regarding unmeasurable, subjective working qualities.
ISO 1559 1st ed. states:
Silver: 65% min. Tin: 29% max. Copper: 6% max. Mercury: 3% max. (So called pre-amalgamation) Zinc: 2% max. One of the drawbacks identified in conventional amalgam is the instability of the metal in terms of corrosion leaving a black, tarnished surface. Corrosion-products of amalgam without contact with other metals does not contain mercury (25). Tin is however one of the most probable metals to form corrosion products. As a consequence mercury is liberated when the gamma-two phase corrodes.
Another drawback is the suspicion that conventional amalgams high creep values are responsible for an increased incidence of marginal fractures.
Conventional amalgams are also subjected to the formation of droplets on the surface of severely deformed metal - a subject to be discussed later. This formation is however almost negligible as compared to modern non-gamma-two amalgams.
Non-gamma-2 amalgams
Corrosion was identified as one of the major drawbacks of conventional amalgam. This corrosion mainly takes place in the gamma-two phase. In 1963 a new composition of dental amalgam was described by the Canadians Prof. Innes and Prof. Youdelis at the University of Windsor (26). This amalgam is commercially known as Dispersalloy ® and has been followed by a number of similar amalgams (4).
Considerable amounts of copper were once again introduced - the amounts were however not as great as in the old copper amalgams. The result of this new formula was a great reduction in gamma-two phase. As a bonus the material also became stronger, partly due to the e-phase and the h'-phase. These phases are alloys of copper and tin. In other words - modern non-gamma-two amalgams contain bronze.
As previously mentioned the composition of dental amalgams is regulated in the ISO standard 1559 (24, 27). It is interesting to notice that the new non-gamma-two amalgams did not fulfil the requirements of ISO 1159 first edition - the copper content was too high (24). At the time of the introduction of this standard these new amalgams had already hit the market. Eight years later, following the success of the new amalgams, the standard was re-written allowing the use of the non-gamma-two amalgams. In other words the standard did not regulate the market - in fact the reverse was the case.
The new standard - ISO 1559 ed. 2 - "updating of the composition requirements to include alloys with high copper contents" (27):
Silver: 40% min. Tin: 32% max. Copper: 30% max. Mercury: 3% max. (So called pre-amalgamation) Zinc: 2% max. In this new standard stability testing is still lacking. It says however: "It is proposed to include a corrosion test requirement for dental amalgam at the earliest possible date. Such a test may include ion release or gravimetric loss of substance after leaching completely hardened amalgam specimens in a suitable corrosion solution". Testing of stability in terms of mercury vapor is not mentioned.
According to the new standard inclusion of other ingredients than stated are permitted provided that "the manufacturer presents adequate evidence of clinical and biological investigations in accordance with ISO/TR 7405 to show that the alloy with the deviation in composition is safe to use in the mouth".
ISO/TR 7405 (technical report - not standard) provides a set of investigations to make a biological assessment of a dental material. The manufacturer is free to choose what test or tests he considers appropriate out of the seventeen described (28). This technical report contains a "grandfather clause" allowing materials on the market before 1984 to be used without testing: "Materials certified by national and international agencies at the time of publication of this Technical Report shall be assumed to be acceptable unless there is evidence indicating a reasonable doubt". The bulk of scientific investigations questioning the use of dental amalgam and national restrictions against the use of it has until now not been considered "reasonable doubt".
To Formation of droplets on the surface of non-gamma-two amalgams
On Reality. Publisher and editor: Bo Walhjalt. ISSN 1650-9323.
© Bo Walhjalt and authors. | Comments on this page
Latest update 2002-12-05original url of this page: http://www.gbg.bonet.se/bwf/art/instab/types.html
