![]()
On the Instability of Amalgams
![]()
The symbiosis between the dental and industrial communities and their scientific journals
![]()
On the Instability of Amalgams...cont. -
Increased mercury vapor emission from modern non-gamma-two amalgams
Following the findings of deposits on the surface of non-gamma-two amalgams the logical question is if this phenomenon is linked to an increase in the emission of mercury vapor. Initial experiments showed that both conventional and non-gamma-two amalgams emitted easily measurable amounts of Hg-vapor. In both cases a dramatic increase in the emission could be observed when a piece of cotton wool was gently slided over the surface. A fragile layer of passivating nature was suspected.
Hg-vapor is regarded as the most concerning toxic substance emitted fromdental amalgam. It is indeed surprising that dental science has not looked for a link between the formation of droplets and an increased emission of mercury vapor. As mentioned before no scientific article has been published on the extraordinary phenomenon of droplets on modern amalgams so relevant for the understanding of this material.
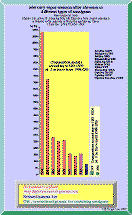
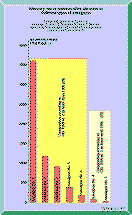
Figure 2 Figure 3 Click in the image to view full size! In 1988 a project was initiated at the department of chemistry at Linköping University, Sweden. It was performed by Cecilia Toomväli as a part of her Masters Degree in chemistry. The paper is unfortunately written in Swedish but with an abstract in English (41):
"The release of mercury vapor from dental amalgams has been studied. The purpose has been to examine if there is a fragile passivating oxide layer on the surface of the amalgams in air. To find out if there is an oxide layer the amalgams have been exposed for a certain loading. The mercury vapor released has been detected in different gases as air, argon and nitrogen. Different parameters as time and temperature have been varied. Another purpose has been to find out if there are any differences among the five types of amalgams that have been tested.
The results indicate that a fragile, passivating oxide layer is built up in air and that oxygen is adsorbed on the surface. The investigation also shows a difference in mercury release between different amalgams. The non-gamma-two amalgams with high copper content have a greater release of mercury vapor than conventional amalgams."
Dental amalgams have been used for more than 160 years (42). It took however until the 1994 before any scientist found one of the mechanisms behind the emission of mercury vapor from dental amalgams. Mahler, Adey and Fleming has shown that the emission of mercury vapor is related to the amount of tin in the gamma-1 phase (8). As can be seen from Figure 2 it is the modern non-gamma-two amalgams that are at the top of the list.
In a study by Berglund the author concludes: "In air during cyclic dipping into the aqueous media, the conventional amalgam specimens released mercury vapor at lower rates over the whole period than did the dispersed and single-composition types studied"(43). They also included an in vivo investigation into their study but failed to get any useful measurements in subjects with non-gamma-two amalgams as well as in those with conventional amalgams.
Boyer says: "High-copper, admixed amalgam emitted higher amounts of mercury vapor than the conventional amalgam under the experimental conditions of this study" (44).
Psarras et al say: "High copper, non-gamma 2-amalgams, released more mercury vapor than the conventional one throughout the experiment" (45).
Ferracane et al has performed investigations on a number of experimental amalgams with different amounts of gamma-one tin (46). Amalgams with compositions according to the new standard ISO 1559 ed. 2 emits considerably more mercury vapor than those with ingredients in accordance with the old ISO 1559 ed. 1, see Figure 3.
In his long term corrosion studies of amalgam Moberg says: "In general, the high copper amalgams released more corrosion products into the solution than the conventional one" (47). In his doctoral dissertation he concludes: "Placing the conventional amalgam in contact with the blended high-Cu amalgam caused an increased amount of Cu in the solutions probably due to the easily corrodable Cu-phases of the high-Cu amalgam. The high release of Cu from high-Cu amalgams is in accordance with earlier results (Espevik 1977)" (48). He also states that: "The amounts of corrosion products increased exponentially with the corrosion depth".
Non-gamma-two amalgams were introduced on the market to increase strength and corrosion resistance. Decades later when relevant scientific work has been done on this material it is obvious that a giant sub optimization has taken place. Strength and corrosion resistance have been paid for by means of a drastic increase in emission of mercury vapor - the form of mercury regarded as the most hazardous emitted from dental amalgam.
To References
On Reality. Publisher and editor: Bo Walhjalt. ISSN 1650-9323.
© Bo Walhjalt and authors. | Comments on this page
Latest update 2002-12-05original url of this page: http://www.gbg.bonet.se/bwf/art/instability/increased.html